Identification of first-in-class, potent and selective CK1α degrader
In a new study, which was published today in Nature Communications, investigators from St. Jude Children’s Research Hospital describe the discovery and characterization of a novel, selective degrader of casein kinase 1A1 (CK1α) [1]. The small molecule is a potential starting point for the development of therapies for myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML). Oncolines’ scientists contributed to the work by Oncolines® cancer cell panel profiling experiments and bioinformatics analysis.
Targeted protein degradation via PROTACs or molecular glues is a novel and promising drug discovery strategy. One approach uses small molecules that alter the selectivity of cereblon, the substrate recognition domain of the E3 ubiquitin ligase CRL4CRBN. This mechanism of action is exemplified by lenalidomide (Revlimid®), a blockbuster drug developed by Celgene (now part of BMS) used for treatment of various hematological malignancies, including multiple myeloma and MDS. Lenalidomide induces the degradation of several proteins, including CK1α, which is involved in regulation of the p53 pathway [2]. Interestingly, although the clinical efficacy of lenalidomide in MDS has been attributed to the degradation of CK1α, the compound induces only modest, incomplete degradation of CK1α at very high concentrations [2]. Dr. Gisele Nishiguchi, Dr. Zoran Rankovic and their colleagues at St. Jude’s hypothesized that more complete CK1α degradation could result in greater efficacy, and therefore set out to identify novel and potent selective CK1α degraders.
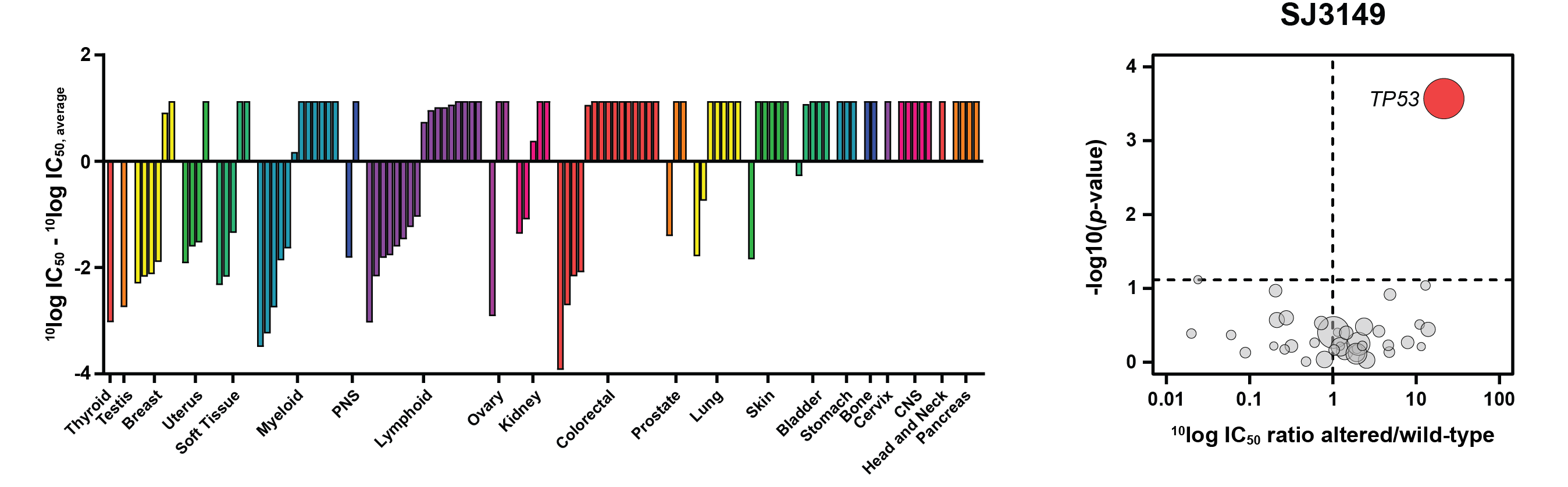
Through screening of a proprietary library of cereblon ligands and structure-guided medicinal chemistry optimization, Nishiguchi et al. developed SJ3149, a potent and selective CK1α degrader, SJ3149 [1]. This compound inhibited the proliferation of a range of AML cell lines, including MOLM-13 in which lenalidomide is inactive, indicating potential application in AML. Furthermore, in Oncolines® profiling experiments with 115 human cancer cell lines, SJ3149 showed activity across a broad range of tissues and diseases, while still retaining selectivity (Figure A). Bioinformatics analyses using the SJ3149 drug-response profile demonstrated that SJ3149 most potently targets cell lines that are wildtype for the TP53 gene (Figure B). This suggests that active p53 signaling is required for the anti-proliferative effects of SJ3149. Finally, correlation analysis of the cellular SJ3149 drug-response profile with an Oncolines® library of 120 pre-profiled compounds revealed a significant correlation with the MDM2 inhibitor nutlin-3a, confirming the activity of SJ3149 in p53-active cell lines.
The study provides a rationale for the application of selective and potent CK1α degraders across a wide range of hematologic and solid tumors. Additionally, the study shows that the selectivity and potency of degraders can be rationally optimized.
Figure: SJ3149 activity in a panel of 115 cancer cell lines. Left: Waterfall plot showing the ranking of drug response based on SJ3149 IC50 values of the profiled cell lines. Cell lines were grouped and colored according to their primary tissue origin. Right: Volcano plot comparing SJ3149 IC50 differences between altered and wild-type cell lines for 38 established cancer genes. The red node indicates significantly higher IC50 in the TP53-altered cell lines.
References
- [1] Nishiguchi et al. (2024) Selective CK1α Degraders Exert Antiproliferative Activity Against a Broad Range of Human Cancer Cell Lines. Nature Communications 15, 482
- [2] Krönke et al. (2015) Lenalidomide induces ubiquitination and degradation of CK1α in del(5q) MDS. Nature 523, 183–188.
Oncolines B.V. is a precision medicine services company in oncology and cancer immunotherapy. Oncolines is part of the Symeres group of companies, a group of high-quality CROs and CDMOs based in Europe and the United States.