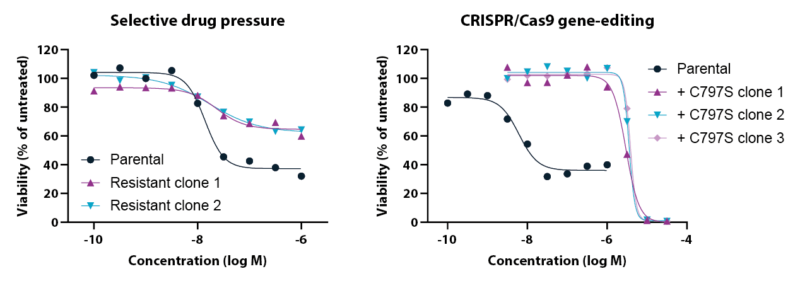
Mutations in the epidermal growth factor receptor (EGFR) are the most common oncogenic drivers in non-small cell lung cancer (NSCLC), but many patients treated with EGFR kinase inhibitors develop drug resistance. At Oncolines, we have more than 70 human cancer cell lines available that are selected for resistance against various targeted therapies, including many EGFR inhibitor-resistant cell lines. These cell lines can be applied in proliferation assays, drug combination studies and mechanism-of-action studies.
At the AACR Annual Meeting, we will present new data on osimertinib-resistant cell lines. Osimertinib is a third-generation EGFR tyrosine kinase inhibitor that irreversibly binds to active-site cysteine 797 (C797) and was originally developed to target resistance against first- and second-generation inhibitors. Owing to its efficacy and selectivity, osimertinib is now used as first-line treatment for NSCLC patients with the activating EGFR mutation L858R or exon 19 deletion. The most frequently occurring on-target resistance mechanism against osimertinib is the C797S mutation, which has urged the development of fourth-generation EGFR inhibitors. In collaboration with our partners Carna Biosciences, Inc. (Kobe, Japan) and Pangaea Oncology S.A. (Barcelona, Spain) we have compared osimertinib and the three fourth-generation EGFR inhibitors BDTX-1535, BLU-945 and JBJ-09-063 in biochemical and cell-based assays.
The poster (abstract #4668) will be presented on Tuesday morning, April 9th from 9:00 to 12:30 (PST) and can be found in section 27, at poster board 21.
Oncolines B.V. is a precision medicine services company in oncology and cancer immunotherapy. Oncolines is part of the Symeres group of companies, a group of high-quality CROs and CDMOs based in Europe and the United States.