June 23, 2020: NTRC presents the poster #5604, titled ‘High TDO and IDO1 expression in ovarian cancer-associated cells isolated from malignant ascites’ at the AACR Virtual Annual Meeting II.
High TDO and IDO1 expression in ovarian cancer cells isolated from malignant ascites
Oss, June, 23, 2020 – NTRC presents at the AACR Virtual Annual Meeting II, June 22-24, 2020. The poster (#5604) shows that high surface expression of PD-L1, and/or high expression of IDO1 or TDO2 was observed in several ovarian cancer-associated cell samples isolated from malignant ascites.
In epithelial ovarian cancer (EOC), 15-20% of tumors do not respond to first-line chemotherapy, consisting of platinum-based therapy plus paclitaxel, and in recurrences the response rate is even lower. Clinical trials with immunotherapies, such as PD-1/PD-L1 blockade, have so far not been successful. There is a great need of novel therapies with improved long term treatment outcome, as well as diagnostic tools and biomarkers to predict chemotherapy response in the clinic.
We have used malignant ascites collected from EOC patients, to identify biomarkers of chemotherapy response and to develop assays to support drug discovery programs on TDO and IDO1 with clinically relevant assays.
Ascites was gathered from eighteen patients with advanced stage EOC. Cells were isolated by centrifugation and tumor cells were separated from immune cells by overnight adherence to tissue culture plates. Both cell fractions were characterized for the expression of immune and cancer cell markers by flow cytometry and quantitative PCR. TDO and IDO1 activity was determined using a functional assay and small molecule inhibitors.
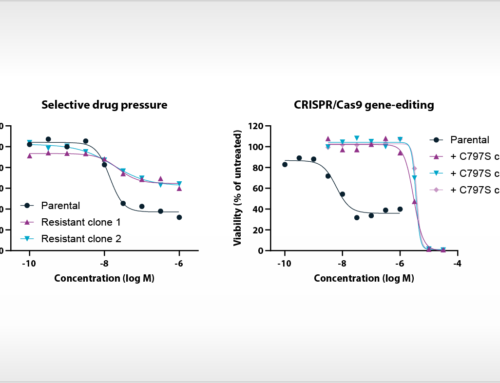
All samples expressed the ovarian cancer markers CA125, HE4, or both. Flow cytometry analysis of eleven adherent cell samples with anti-EpCAM or CA125 antibodies confirmed their EOC origin. Five of six samples showed high cell surface expression of PD-L1. Adherent cell samples were characterized for TDO and IDO1 expression and activity. Two samples out of eighteen showed high IDO1 gene expression. However, only after stimulation with IFNγ, the tryptophan metabolizing activity in these samples was detectable, and could be inhibited with selective IDO1 inhibitors. In contrast, four samples expressed relatively high TDO and detectable endogenous tryptophan metabolizing activity, which could be inhibited by selective TDO inhibitors. The immune cell composition of the ascites, the expression and activity of TDO, IDO1 and PD-L1 were related to the clinical outcome of the patients from which the ascites was derived.
EOC-associated cells isolated from malignant ascites were used to develop functional assays to support drug discovery programs on TDO and IDO1.
NTRC is a precision medicine company dedicated to discovering new anti cancer drug candidates. We help you to find a mechanistic hypothesis before entering the clinic. We can study a wide range of cancer cells, primary patient material and immune cells in vitro, in isolation and in coculture, after exposure to monotherapy and combination therapy. In addition, we perform in-depth mechanistic analyses in cells and by biophysical methods, such as Biacore and LC-MS/MS. Keywords are: Quality. Flexibility. Short Turnaround Time.